Boiling Point Of Oil
/SPR_1328753-smoking-points-of-fats-and-oils-vegetableoil-5c7989d3c9e77c00012f81e7.png)
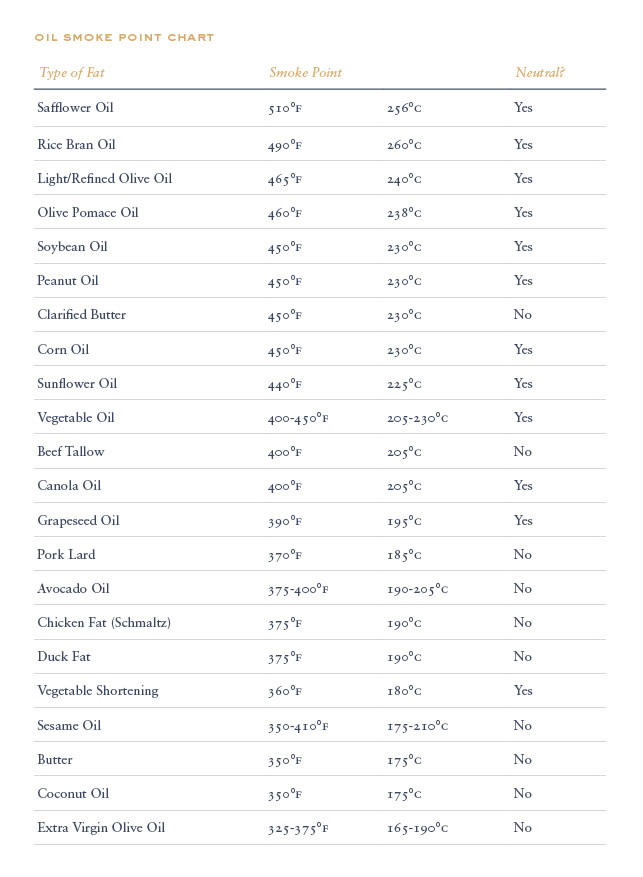
When choosing a fat or oil to cook with the most important temperature to consider is the smoke point well before a cooking fat or oil reaches its boiling point it will begin to smoke.
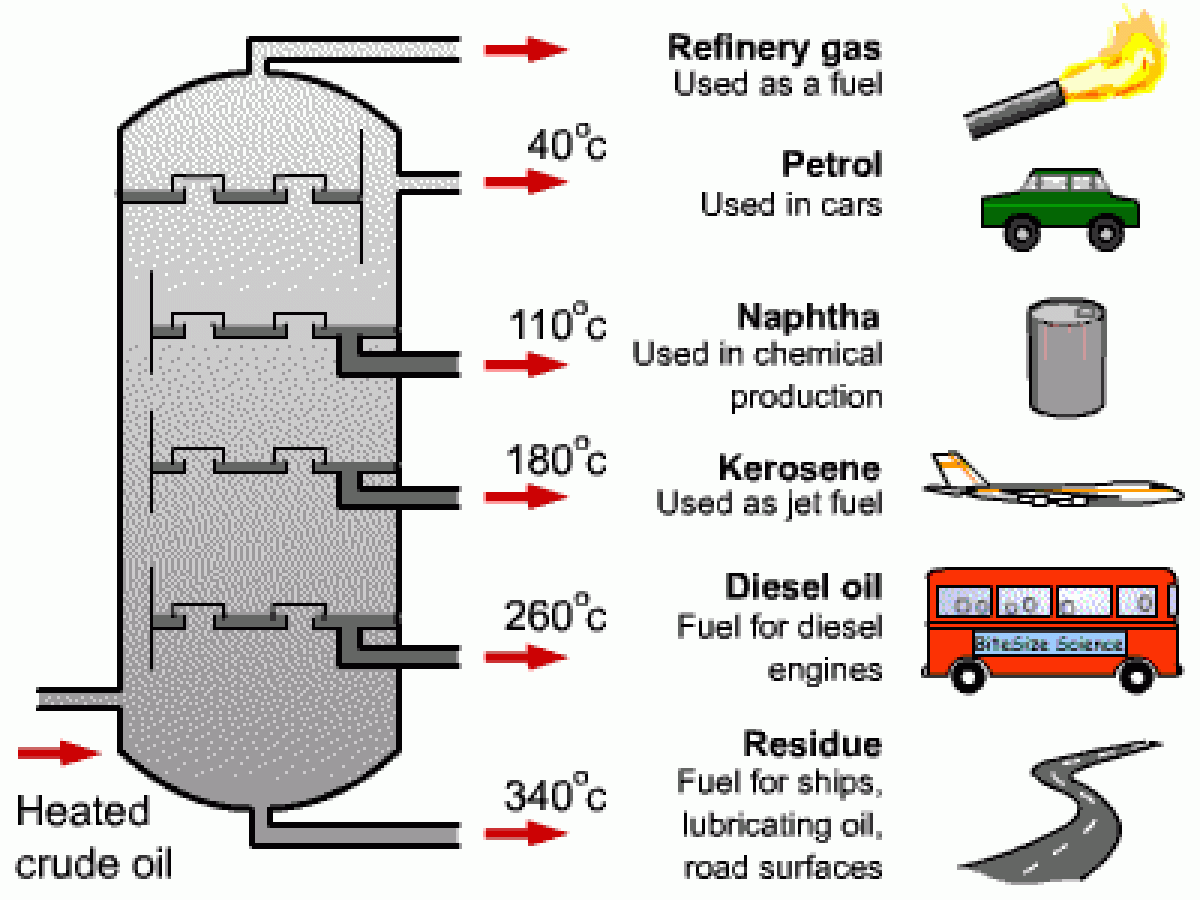
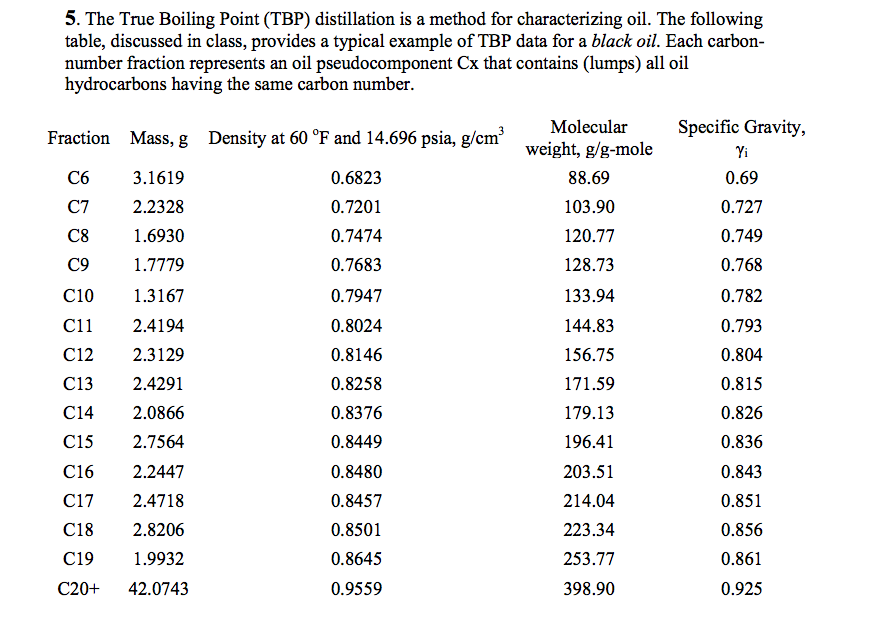
Boiling point of oil. The boiling point of oil depends upon the specific type of oil that is being heated as well as its specific purity. Once any oil begins to smoke it starts to break down altering its flavor and releasing free radicals. The smoke point also referred to as the burning point is the temperature at which an oil or fat begins to produce a continuous bluish smoke that becomes clearly visible dependent upon specific and defined conditions. Crude oil subjected to refining involves a spectrum of different boiling points to extract the various elements comprising it.
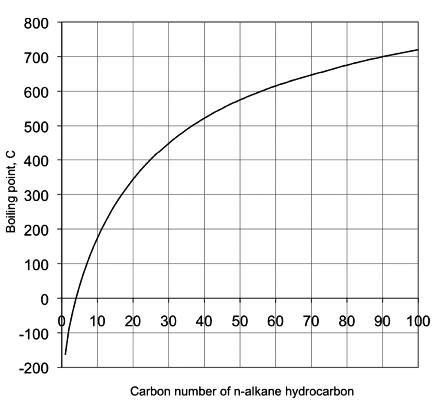
Other cooking oils have similar boiling points. The astm d86 and d1160 standards describe a simple distillation method for measuring the boiling point distribution of crude oil and petroleum products. You can compare this to the boiling point of water which is 100 c or 212 f. Fractional distillation of crude oil the boiling point is the temperature at which the vapour pressure of the substance is equal to the atmospheric pressure the boiling point depends on the pressure whereas the pressure increases the boiling point increases.
The boiling point of a liquid is the temperature where the liquid will change into a gas. But more importantly once an oil exceeds its flash point harmful compounds are released that have been linked to myriad health issues. That can result in food that tastes burnt even if it looks perfectly fine. A substance called acrolein makes the oil taste burnt and bitter which can quickly ruin a dish.
Fat quality smoke point. The boiling point of soybean oil the most common cooking oil typically marketed as vegetable oil is approximately 300 degrees celsius. Using astm d86 boiling points are measured at 10 30 50 70 and 90 vol distilled. The boiling range for crude oil may exceed 1000 f.
/SPR_1328753-smoking-points-of-fats-and-oils-vegetableoil-5c7989d3c9e77c00012f81e7.png)